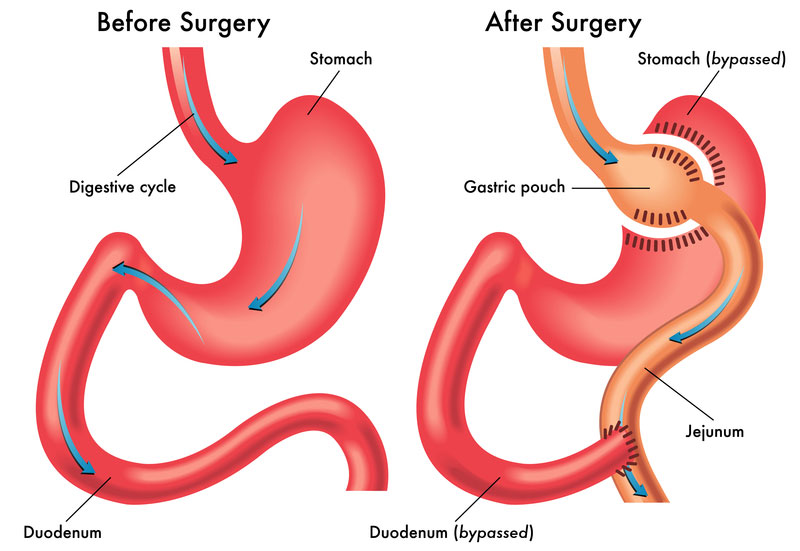
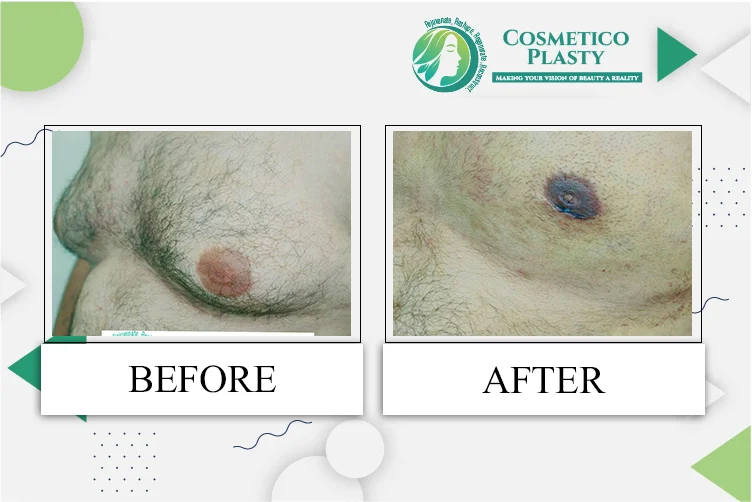
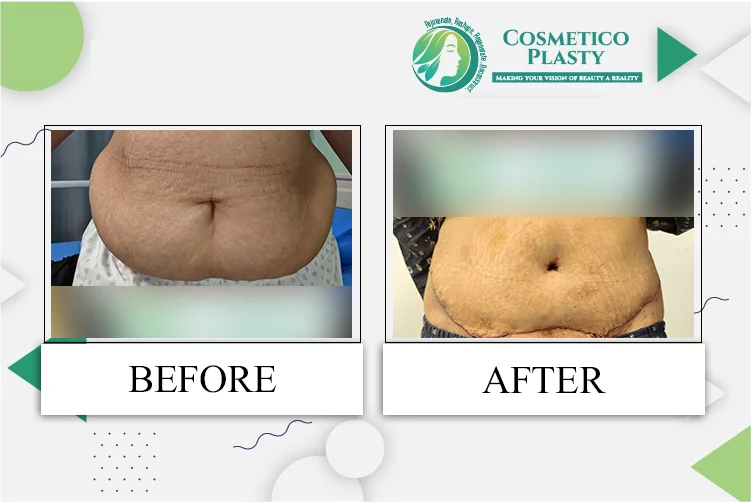
Pharmacovigilance in New Zealand – DDReg Pharma
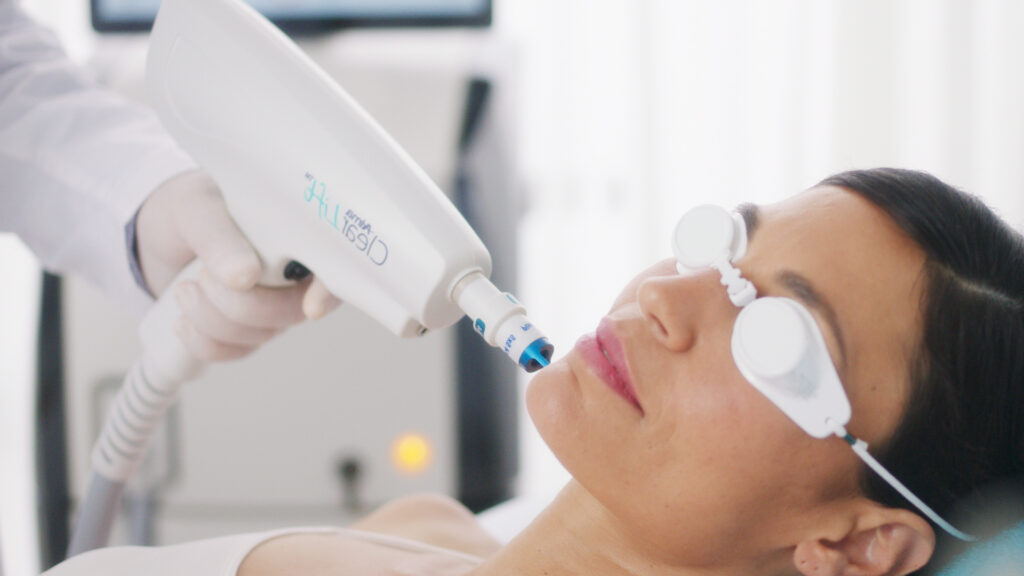
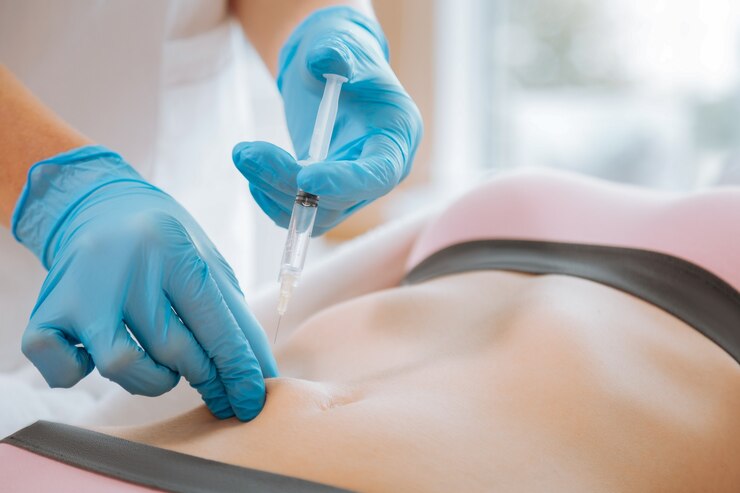
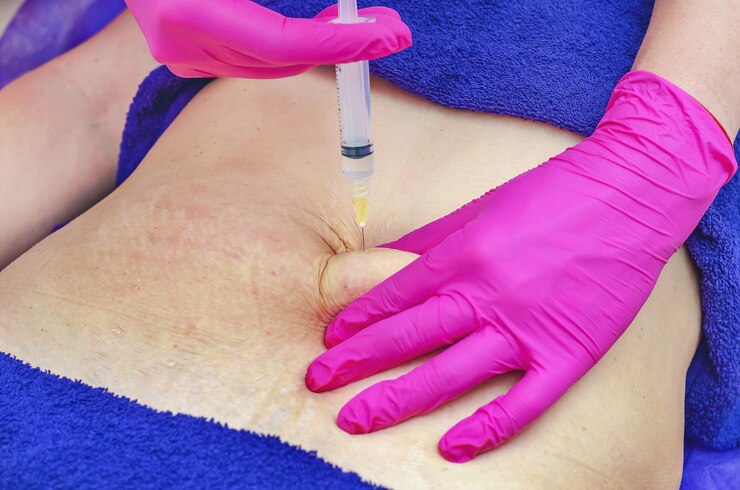
Medsafe is responsible for regulating pharmaceutical products in New Zealand and operates under the Medicines Act of 1981, which requires sponsors to report any significant adverse effects of their medications. The Guidelines on the Regulation of Therapeutic Products in New Zealand” (GRTPNZ) outlines this requirement and also includes a Pharmacovigilance (PV) guidance document. This document […]


 English
English