What Are the Key Elements of Patient-centric Clinical Trials?

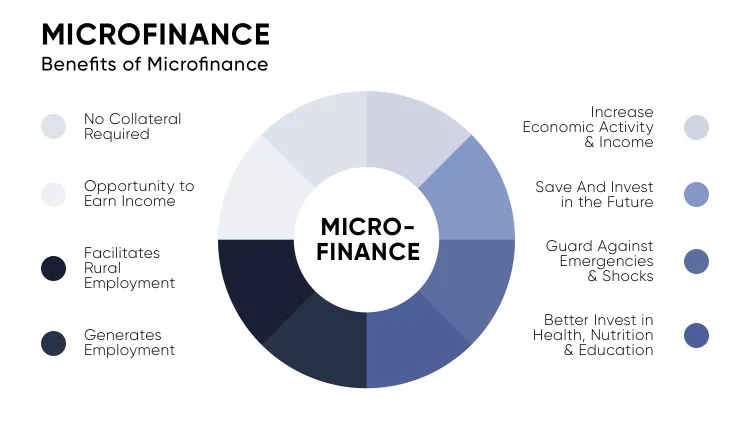
Patient-centric clinical trials place patients at the heart of the research process. They start with active patient engagement, inviting participants to help shape the study design and decisions, ensuring the research truly reflects their needs. Flexible trial designs—such as decentralized models and remote monitoring—make participation easier and less burdensome.
Clear communication throughout the trial builds trust by simplifying complex details into straightforward, understandable information. Personalized support, tailored to individual needs, ensures that each patient feels valued and well-guided during every step of the process. Ultimately, by focusing on patient outcomes, these trials measure success not just by clinical data but by real improvements in quality of life. Key Elements of Patient-Centric Clinical Trials include but are not limited to the below listed:
- Patient Engagement
- Flexible Trial Designs
- Clear Communication
- Personalized Support
- Focus on Patient Outcomes
-
Patient Engagement and Communication
- Building Trust through Transparent Communication
- Interactive Platforms
- Regular Updates:
-
Accessibility and Convenience
- Travel and Logistics Support: For patients who may face challenges attending frequent site visits, providing travel assistance, reimbursement for transportation, or even home healthcare options can significantly reduce barriers to participation. Some trials even provide telehealth consultations, meaning patients don’t need to travel long distances for every follow-up visit.
- Decentralized Trial Models: Advances in telemedicine and digital health are enabling decentralized trials where patients participate in the study from their homes, providing greater flexibility. Decentralization can help increase recruitment and retention, especially for underserved or remote populations.
- Flexibility in Participation: To account for patients’ real-world challenges, flexibility in treatment regimens, monitoring methods, and visit schedules is key to reducing the burden of participation. Trial sponsors can explore options such as remote monitoring, virtual consultations, and at-home testing kits.
-
Patient Support and Education
- Holistic Support Services: Beyond medical care, patient support services can include mental health support, peer-to-peer groups, and wellness programs to ensure patients feel emotionally supported. This could be particularly crucial for long-term or life-threatening conditions, where patients experience both physical and emotional strain.
- Patient Education Tools: Providing patients with easily understandable, multimedia resources about their condition, treatment options, and the trial process in various formats (videos, infographics, podcasts, etc.) can help empower them to take an active role in their care.
- Cultural Sensitivity and Language Accessibility
4. Personalized Care
- Tailored Treatment Plans: Implementing individualized care plans that reflect the diversity of patients’ needs can improve the efficacy of a clinical trial. This might involve adjusting doses based on patient characteristics (e.g., age, comorbidities), or offering alternative treatment routes if a patient experiences intolerable side effects.
- Integration with Routine Care: Facilitating the integration of the trial protocol into a patient’s existing care routine or current treatment regimen helps to minimize disruptions to their daily life. Clinical trial coordinators can collaborate closely with patients’ primary care providers to ensure alignment between the trial and routine care.
-
Real-Time Data Collection
- Remote Patient Monitoring Technologies: The use of wearable devices, biosensors, and mobile apps enables continuous monitoring of patient data such as vital signs, blood glucose, sleep patterns, or physical activity. This data not only allows researchers to track treatment efficacy in real-time but also helps identify any issues early, minimizing risks to patients.
- Patient-Reported Outcomes (PROs): Leveraging technology to allow patients to self-report their symptoms, quality of life, and treatment side effects through digital tools helps provide a richer, more accurate picture of the trial’s impact on their day-to-day lives.
- Data Integration for Better Decision Making: Collecting and integrating real-time data allows for more adaptive trial designs, where adjustments can be made based on patient responses, making the trial more responsive and personalized.
-
Improved Recruitment and Retention
- Broadening Eligibility Criteria: Traditional trials often have restrictive eligibility criteria, which can exclude certain populations. By expanding eligibility criteria (where clinically safe and feasible), more diverse groups can participate. This includes patients from varied age groups, ethnicities, and those with comorbidities.
- Patient Advocacy and Community Involvement: Collaborating with patient advocacy groups or community leaders can improve recruitment by reaching patients who may not be aware of available clinical trials. These groups can also help raise awareness and help patients feel more supported and confident in participating.
- Incentive Programs: Offering incentives, such as financial compensation, travel allowances, or access to treatments post-trial, can help encourage participation and mitigate the financial burden.
-
Collaboration with Patient Advocacy Groups
- Co-Designing Trials: Patient advocacy groups can provide invaluable insights into trial design, from recruitment strategies to outcome measures that matter most to patients. Involving patients in the design phase leads to a more relevant and feasible study structure that aligns with their needs.
- Ongoing Advocacy for Patients: Advocacy groups can help in monitoring patient welfare throughout the trial, ensuring that patients are heard and that their experiences are reflected in the trial’s decision-making processes.
-
Outcome Measurement Relevant to Patients
- Patient-Reported Outcomes (PROs) and Quality of Life: Instead of focusing exclusively on traditional clinical endpoints like biomarkers or disease progression, patient-centric trials increasingly include outcomes that matter to patients, such as symptom management, side-effect profiles, and the overall impact on quality of life.
- Long-Term Benefits: It’s not just about short-term efficacy; patient-centric trials also consider long-term outcomes like sustained remission, durable benefits, or the impact of treatments on a patient’s overall well-being after the trial ends.
-
Post-Trial Support
- Continued Access to Treatment: For trials involving promising treatments, providing access to the intervention after the study ends, especially if it is effective for managing a chronic or life-threatening condition, is an important component of patient-centered care.
- Long-Term Follow-Up: Long-term follow-up can help patients manage their condition post-trial, especially if the trial intervention showed lasting effects. These check-ins also allow researchers to gather data on long-term safety and efficacy.
- Patient Advocacy for Access to Medications: Some clinical trials may offer post-trial access programs or expanded access to drugs that have not yet been approved, allowing patients continued access to the treatment after the trial concludes.
-
Ethical Considerations
- Respecting Patient Autonomy: Patient-centric trials emphasize the importance of ensuring that participation is voluntary and that participants can withdraw at any time without penalty. This autonomy is central to patient trust and collaboration.
- Data Privacy and Security: With the increasing use of digital tools and remote monitoring, ensuring the privacy and security of patient data is paramount. Clinical trials should adhere to data protection regulations such as GDPR (General Data Protection Regulation) and HIPAA (Health Insurance Portability and Accountability Act).
- Transparent Reporting of Results: Ethical considerations extend beyond the trial itself. Once completed, results should be shared transparently, whether the outcomes are positive or negative. This open approach fosters patient trust and encourages future participation.
Explore https://onlinetechlearner.com/ for the latest blogs.
Conclusion:
Incorporating patient-centric elements into clinical trials ensures that the trials are not only scientifically robust but also designed with the patient’s experience, needs, and preferences at the forefront. When patients feel that their well-being is prioritized, they are more likely to stay engaged, provide valuable data, and contribute to the overall success of the trial. This approach helps to advance the development of new treatments that are truly aligned with patients’ needs, making clinical trials more effective and meaningful. Opt for the best Quality assurance in clinical trials to make it easier on yourself eventually.


 English
English 




























































































































































































































































































 Office Clearance for SMEs: Affordable Solutions Businesses
Office Clearance for SMEs: Affordable Solutions Businesses